|
|
|
|
|
|
 |
| Fear, Anxiety and
Anguish | |
|
|
|
|
|
| ANXIETY
NEUROTRANSMITTERS |
|
|
Because GABA is the primary inhibitory neurotransmitter in
the brain, it obviously plays an important role in controlling
the neuronal hyperactivity associated with anxiety.
Since GABA agonists can induce comas, the pharmaceutical
industry has had to turn to other ligands that enhance GABA’s
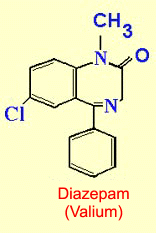
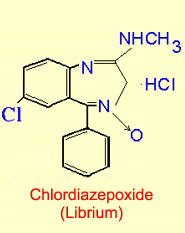
effects. Benzodiazepines
such as Valium and Librium, which act as modulators for GABAa
receptors, have become some of the best anxiolytics
available.
 |
 |
|
In just
the same way that, once scientists had characterized the
receptors for opiates in the brain, they also discovered
natural endogenous morphines that bind to them,
researchers have now identified molecules produced by
the body that bind to exactly the same site on the GABAa
receptor as synthetic benzodiazepines. These endogenous
benzodiazepines, or endozepines, which seem to be
produced chiefly by the glial
cells, have been partially purified in the human
brain. The term “endozepines” designates both
diazepam-binding inhibitor (DBI) and the peptides
derived from it, including triakontatetraneuropeptide
(TTN) and octadecaneuroepetide (ODN).
|
Despite the potential importance of endozepines as
endogenous ligands for the benzodiazepine site on GABAa
receptors, very little research has been done on the role of
these peptides. Endozepines may of course achieve some of
their effects by modulating GABAa receptors, but other
effects, such as the anorexigenic effect of ODN, might involve
a separate metabotropic
receptor.
DBI may be an inverse agonist for the benzodiazepine
site on the GABAa receptor. In other words, DBI may reduce
the receptor’s chloride permeability and hence GABA’s
effectiveness, and thus would be anxiogenic. In fact, DBI is a
disconcerting molecule for neurobiologists, who have shown far
less enthusiasm about endozepines than they have about
enkephalins and endorphins. Nevertheless, these endogenous
benzodiazepines, through their modulating effects on GABA, are
thought to enable it to play a more flexible role in
neurophysiological processes. It is also thought that
disturbances in their activity may play a role in chronic
anxiety.
|
The molecules conventionally
identified as neurotransmitters are not the only ones
that may have an effect on anxiety. Neuropeptides
such as cholecystokinin (CCK) may also be anxiogenic;
the release of this molecule may be enhanced by
serotonin and norepinephrine in the cortico-limbic
system. The use of cholecystokinin antagonists as
anxiolytics is therefore being considered.
Another peptide, CRH, is a
powerful anxiogenic whose release is stimulated by
stress. Neuropeptide Y has an anxiolytic effect almost
as powerful as the benzodiazepines’. It is believed that
under normal conditions, CRH and neuropeptide Y, through
their opposing effects, constitute a system that
controls the integration of stress signals in the amygdala. | |
|
GABA (gamma-aminobutyric acid) exerts its effects through
at least three different types of receptors: the GABA-A
receptor (which is the best known), and the GABA-B and GABA-C
receptors.
The GABA-A and C receptors are ionotropic,
while the GABA-B receptor is a metabotropic
receptor that modulates the opening of potassium channels
through second messengers involving a G-protein.
|
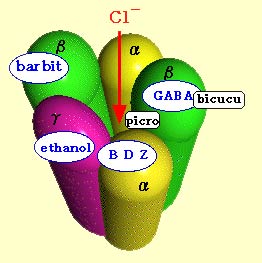
Each of these receptors is a
macromolecular complex comprising several sub-units. For
example, the GABA-A receptor is composed of
5 sub-units surrounding a channel that is
preferentially permeable to chloride ions and to a
lesser extent to bromide ions. The GABA receptor site
appears to be located in the large extracellular domain
of the beta sub-unit. These 5 sub-units have 16
known isoforms, each produced by a different gene.
In addition to the primary binding
sites for GABA, the GABA-A receptor has other secondary
binding sites for molecules that modulate GABA’s
effects, such as benzodiazepines,
barbiturates, convulsants, steroids, and alcohol.
These modulating agents alter
GABA’s efficiency by inducing a change in the protein
architecture of the GABA-A complex. This change modifies
the size of the channel, which in turn modifies the
receiving neuron’s permeability to chloride ions. Since
chloride ions are negatively charged, when they enter
the neuron, they hyperpolarize it. |
|
|
The result is an inhibition of neuronal activity and a
general anxiolytic effect.Treatment with benzodiazepines thus
helps to reduce anxiety by potentiating the effect of GABA,
and more specifically, by making the chloride channel open
more frequently. However, in the absence of GABA at the
primary site on the GABA-A receptor, the modulating molecules
have no effect on the neuron’s chloride permeability.
|
|
|